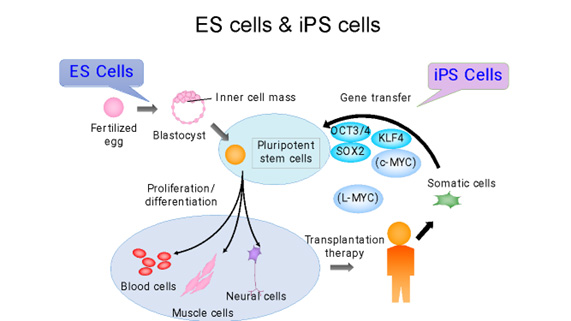
Initially, iPS cells were created from skin cells, but subsequent studies have shown that they can be generated from various types of cells. Nowadays, it’s more common to derive them from blood cells, making the process as simple as drawing blood. The genes used to induce iPS cells have also been modified, and the methods used to introduce these genes have evolved from retroviruses to plasmids and Sendai viruses. Today, iPS cells can even be created using mRNA. However, it’s important to note that iPS cells form a heterogeneous group, meaning they can greatly differ from each other. Even when generated using the same process, individual variations can lead to significant differences in gene expression levels. Moreover, the same cell line can behave differently depending on the cell culture techniques used. Even when following the same protocol, the cells can change differently during maintenance culture and passage, emphasizing the complexity of working with these cells.
Immune Evasion
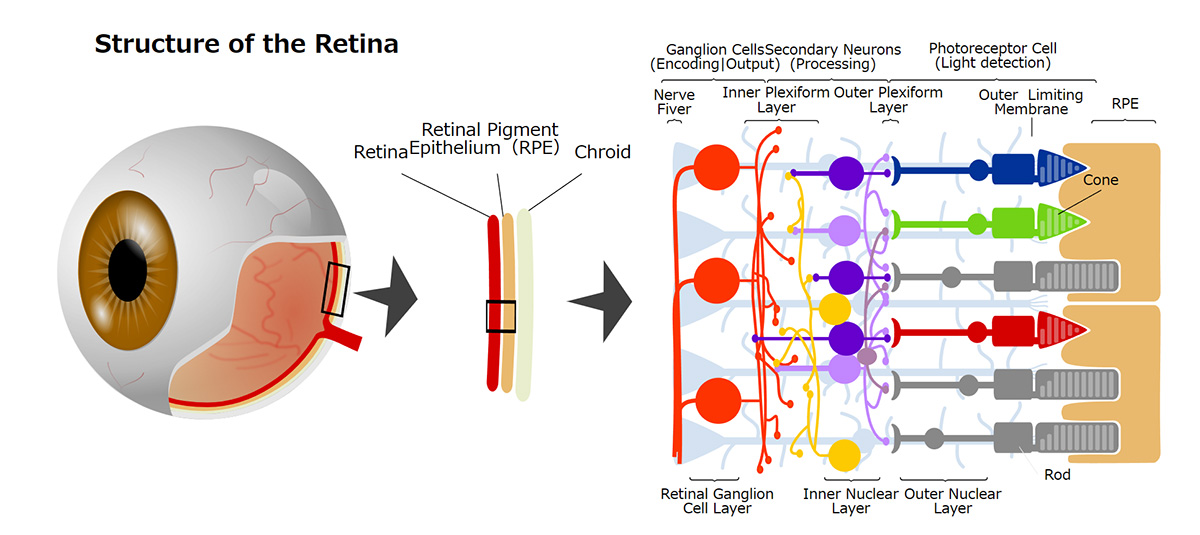
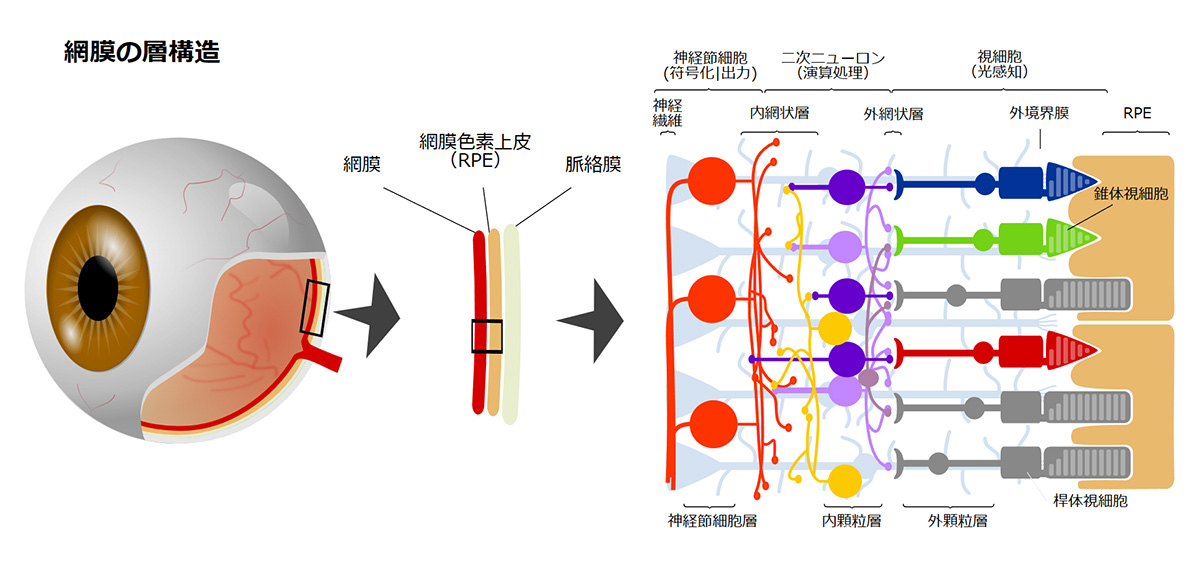
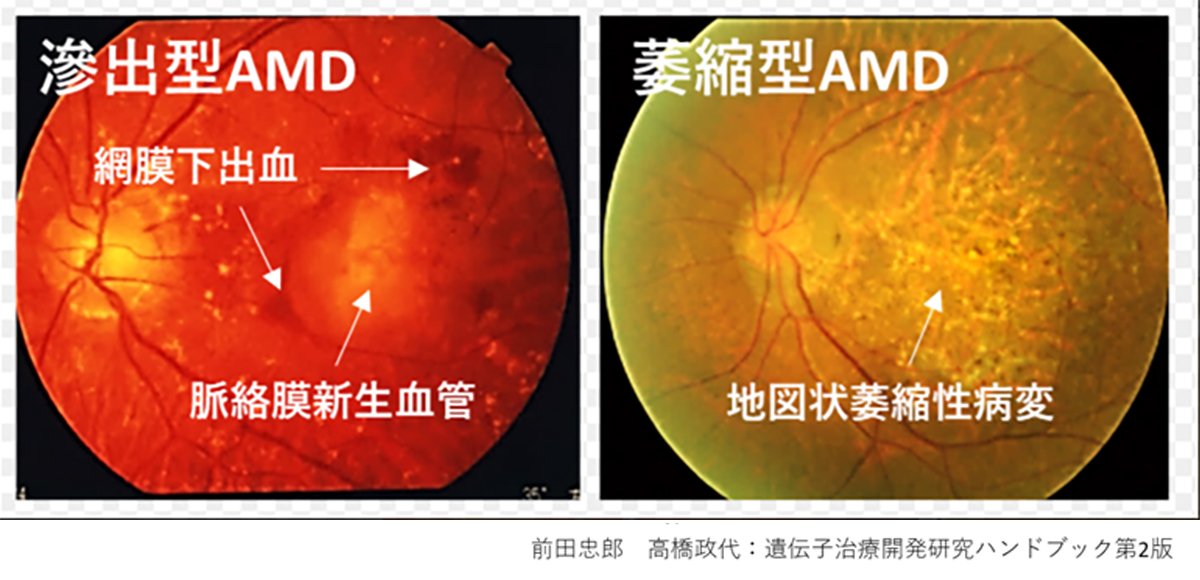
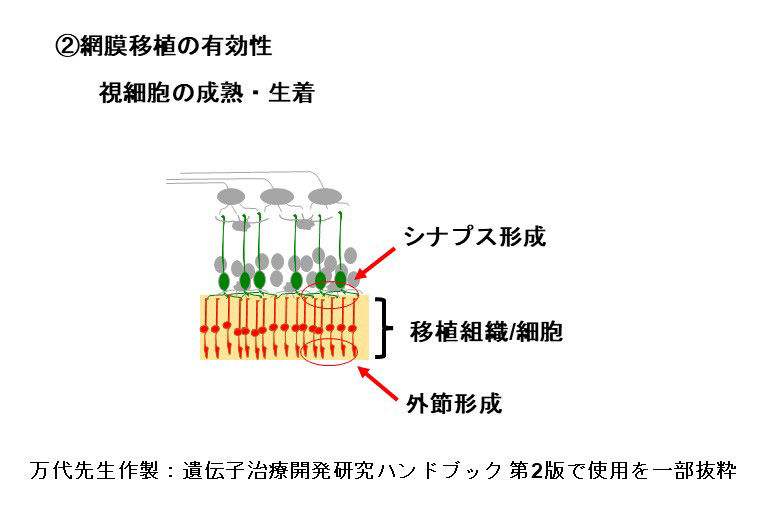
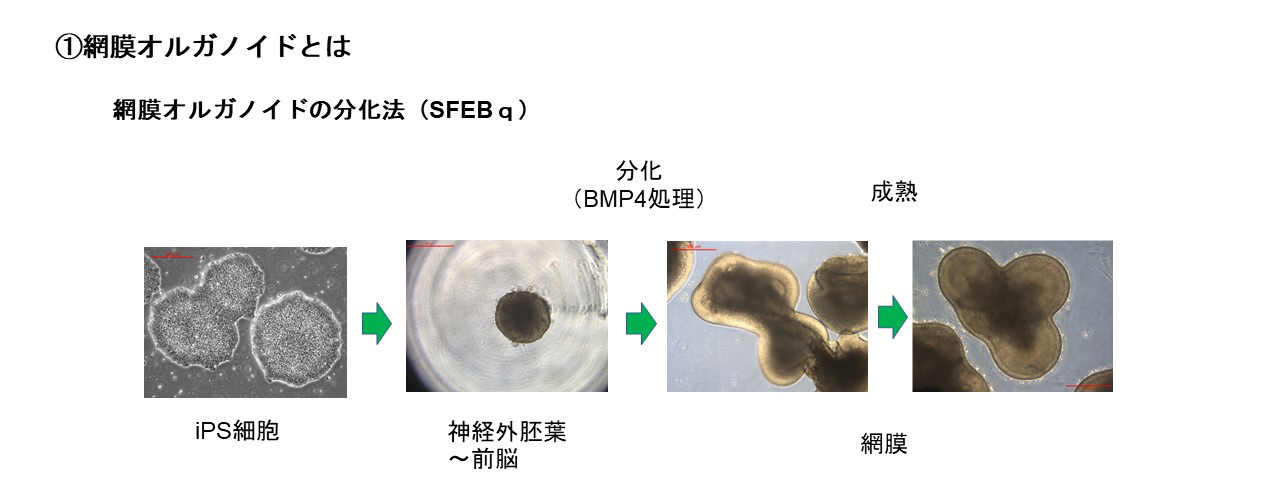
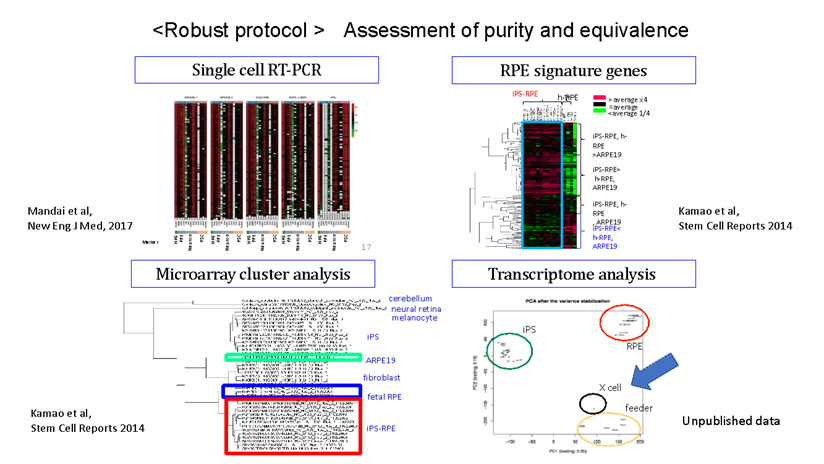
During our pionnering work with iPS cells, we leveraged insights from the broader field of stem cell research to guide the transformation of these iPS cells into Retinal Pigment Epithelial (RPE) cells. While iPS cells are heterogenous and exhibit significant differences even within the same line, when they are differentiated into RPE cells, these differences become insignificant. Historically, there are no instances of these RPE cells turning cancerous, making them a safe choice for our work. We devised a robust method that ensures the creation of consistently safe RPE cells with identical functionalities from each patient’s unique iPS cells(1). This strategic approach made it possible for autologous transplants, effectively bypassing potential issues of immune rejection.
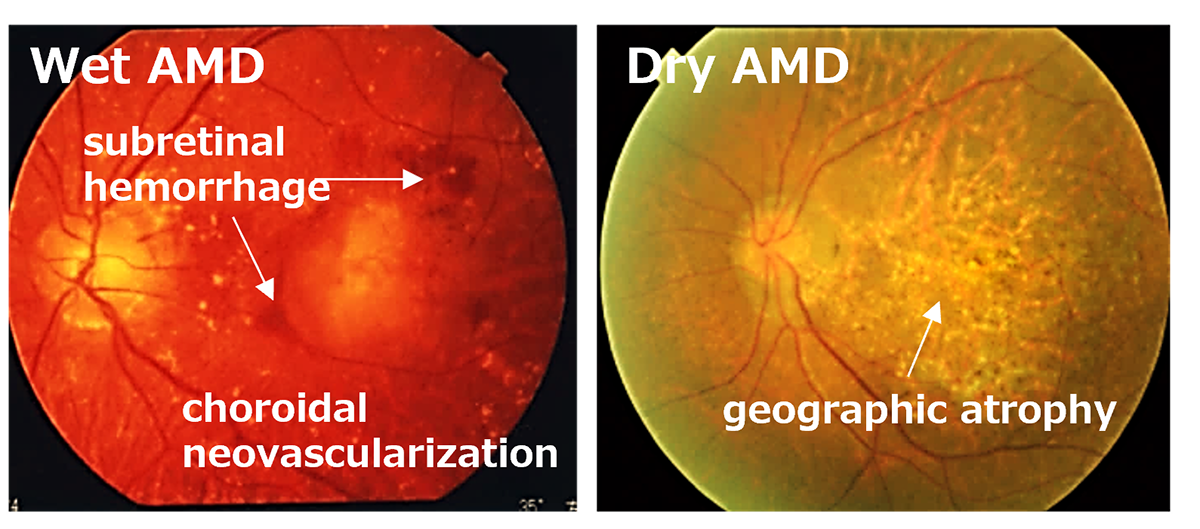
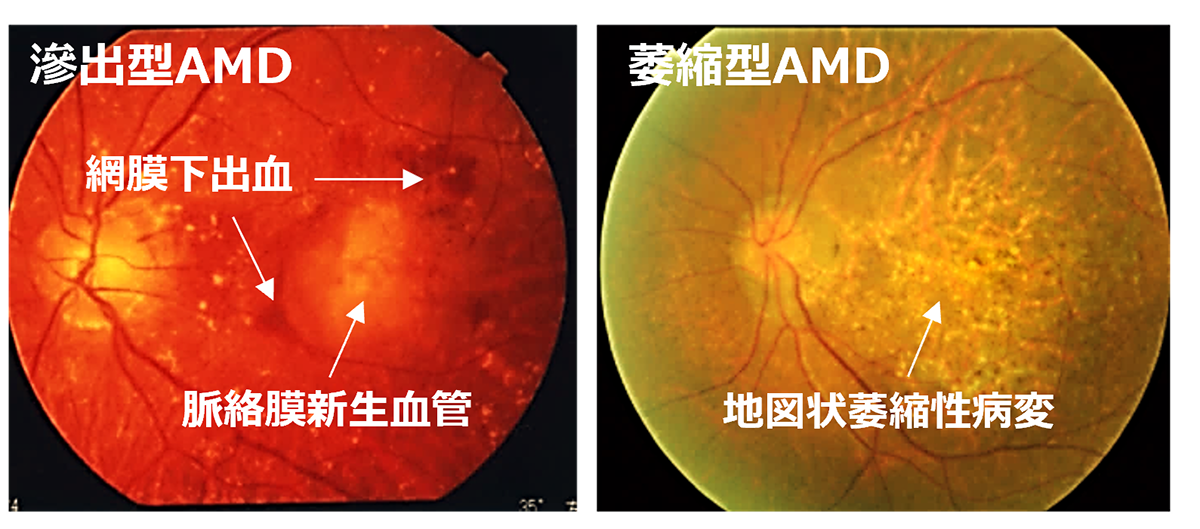
Subsequently, utilizing the iPS cells created by the Center for iPS Cell Research and Application (CiRA) at Kyoto University that matched the 6-locus HLA of a patient, we derived RPE cells and successfully transplanted them into the retina of an elderly patient with age-related macular degeneration. Notably, this procedure was carried out without the use of systemic immunosuppressive drugs[2]. The findings suggest that, when there is a precise HLA match, potential rejection reactions can be efficiently controlled through local steroid administration alone.
Exploiting the insights gained from these clinical trials, we are currently developing treatments that could allow us to transplant cells into any patient without requiring immunosuppressive drugs. This involves creating RPE cells from iPS cells that have undergone genetic alterations to partially disable specific HLA genes, thereby creating a universal cell line applicable to all patients.
Reference:
(1)Characterization of human induced pluripotent stem cell-derived retinal pigment epithelium cell sheets aiming for clinical application. Kamao H, Mandai M, Okamoto S, Sakai N, Suga A, Sugita S, Kiryu J, Takahashi M. Stem Cell Reports. 2014 Jan 23;2(2):205-18.
(2)HLA-Matched Allogeneic iPS Cells-Derived RPE Transplantation for Macular Degeneration. Sugita S, Mandai M, Hirami Y, Takagi S, Maeda T, Fujihara M, Matsuzaki M, Yamamoto M, Iseki K, Hayashi N, Hono A, Fujino S, Koide N, Sakai N, Shibata Y, Terada M, Nishida M, Dohi H, Nomura M, Amano N, Sakaguchi H, Hara C, Maruyama K, Daimon T, Igeta M, Oda T, Shirono U, Tozaki M, Totani K, Sugiyama S, Nishida K, Kurimoto Y, Takahashi M. .J Clin Med. 2020 Jul 13;9(7):2217.

 Kobe City Eye Hospital
Kobe City Eye Hospital Kobe City Eye Hospital
Kobe City Eye Hospital